what is a kill in reference to water
pH of Water
What is pH?
pH is a determined value based on a divers scale, like to temperature. This means that pH of water is not a concrete parameter that can be measured every bit a concentration or in a quantity. Instead, it is a figure between 0 and 14 defining how acidic or basic a body of h2o is along a logarithmic scale ¹. The lower the number, the more than acidic the water is. The higher the number, the more basic it is. A pH of vii is considered neutral. The logarithmic calibration means that each number beneath 7 is 10 times more than acidic than the previous number when counting down. Besides, when counting up above seven, each number is 10 times more basic than the previous number ².
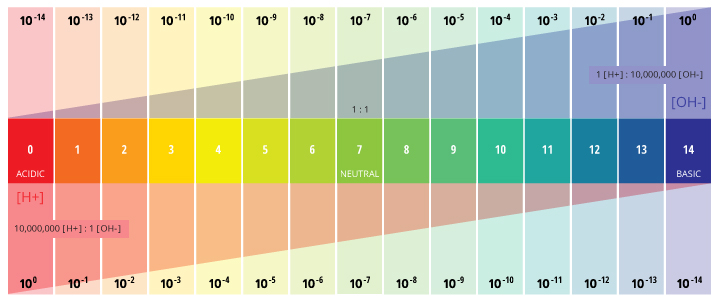
pH stands for the "power of hydrogen" ³. The numerical value of pH is determined by the tooth concentration of hydrogen ions (H+) ³. This is done by taking the negative logarithm of the H+ concentration (-log(H+)). For example, if a solution has a H+ concentration of x-3 M, the pH of the solution will be -log(x-3), which equals 3.
This determination is due to the outcome of hydrogen ions (H+) and hydroxyl ions (OH-) on pH. The higher the H+ concentration, the lower the pH, and the higher the OH- concentration, the higher the pH. At a neutral pH of 7 (pure h2o), the concentration of both H+ ions and OH- ions is 10⁻⁷ M. Thus the ions H+ and OH- are always paired – as the concentration of 1 increases, the other volition decrease; regardless of pH, the sum of the ions volition always equal 10⁻¹⁴ M ². Due to this influence, H+ and OH- are related to the basic definitions of acids and bases.
Acids and Bases
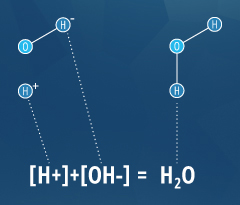
As an operational definition, an acid is a substance that will decrease pH when added to pure water. In the same fashion, a base is a substance that will increment the pH of water ⁴. To farther define these substances, Arrhenius determined in 1884 that an acid will release a hydrogen ion (H+) as information technology dissolves in water, and a base of operations will release a hydroxyl ion (OH-) in water ⁴. Nevertheless, at that place are some substances that fit the operational definition (altering pH), without fitting the Arrhenius definition (releasing an ion). To account for this, Bronsted and Lowry redefined acids and bases; an acrid releases a hydrogen ion or proton (equivalent to H+) and a base accepts a hydrogen ion or proton ⁴. This ways that acids and bases tin can cancel each other out, every bit shown in the water equation to the right.
Basic or Alkaline metal
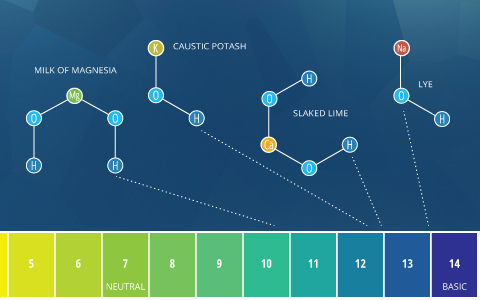
The terms "element of group i" and "basic" mean approximately the same thing. Past the Bronsted-Lowry definition, basic describes any substance that reduces the hydrogen ion concentration and increases the pH of h2o, or in other words, a base of operations ⁴. Alkaline comes from alkali, which refers to ionic compounds (salts) containing alkali metal or alkaline earth metal elements that form hydroxide ions when dissolved in h2o ⁵. Alkali salts are very common and dissolve hands. Due to the hydroxide ions they produce (which increase pH), all alkalis are bases. Some sources define whatsoever soluble base as an alkali ⁵. As such, soluble bases can be described as "bones" or "alkali metal". Withal, insoluble bases (such every bit copper oxide) should only be described equally basic, not alkali metal.
Alkalinity and the pH of Water
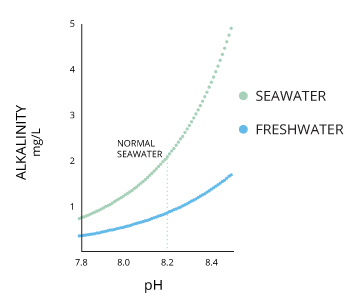
Alkalinity does not refer to alkalis as alkaline does ⁶. While alkalinity and pH are closely related, there are distinct differences. The alkalinity of water or a solution is the quantitative capacity of that solution to buffer or neutralize an acrid. In other words, alkalinity is a measurement of water's ability to resist changes in pH. This term is used interchangeably with acid-neutralizing chapters (ANC) ⁷. If a body of water has a loftier alkalinity, it can limit pH changes due to acid rain, pollution or other factors ⁸. The alkalinity of a stream or other trunk of h2o is increased by carbonate-rich soils (carbonates and bicarbonates) such as limestone, and decreased by sewage outflow and aerobic respiration. Due to the presence of carbonates, alkalinity is more closely related to hardness than to pH (though there are still distinct differences). However, changes in pH can too bear upon alkalinity levels (every bit pH lowers, the buffering capacity of h2o lowers as well) ⁶. pH and alkalinity are direct related when water is at 100% air saturation ⁹.
The alkalinity of water also plays an of import role in daily pH levels. The procedure of photosynthesis past algae and plants uses hydrogen, thus increasing pH levels ¹⁰. As well, respiration and decomposition can lower pH levels. Most bodies of h2o are able to buffer these changes due to their alkalinity, then small or localized fluctuations are apace modified and may exist difficult to observe ¹⁰.
pH and Alkalinity Units
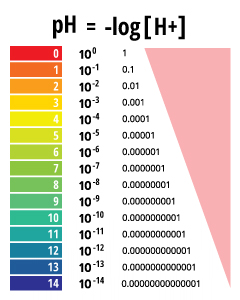
pH values are reported as a number between 0 and 14 as a standard pH unit of measurement. This unit is equivalent to the negative logarithm of the hydrogen ion tooth concentration (-log(H+)) in the solution. Depending on the accuracy of the measurement, the pH value tin can be carried out to one or two decimal places.
However, because the pH scale is logarithmic, attempting to boilerplate ii pH values would exist mathematically incorrect. If an average value is required, information technology can be reported as a median or a range, not every bit a simple calculation ¹⁰.
Alkalinity can exist reported equally mg/50 or microequivalents per liter (meq/50). When in mg/L, it refers to carbonate (CO32-), bicarbonate (HCO3–) or calcium carbonate (CaCO3) concentrations, though calcium carbonate is most common ¹¹.
ane mg/Fifty alkalinity every bit CaCO3 = 0.01998 meg/L alkalinity
1 mg/L alkalinity as CaCO3 = 0.5995 mg/Fifty alkalinity equally CO32-
one mg/L alkalinity every bit CaCO3 = 1.2192 mg/50 alkalinity as HCO3–
Why is pH Important?
If the pH of water is likewise high or also low, the aquatic organisms living inside information technology will dice. pH can too touch the solubility and toxicity of chemicals and heavy metals in the water ¹². The majority of aquatic creatures prefer a pH range of six.5-9.0, though some can live in h2o with pH levels outside of this range.
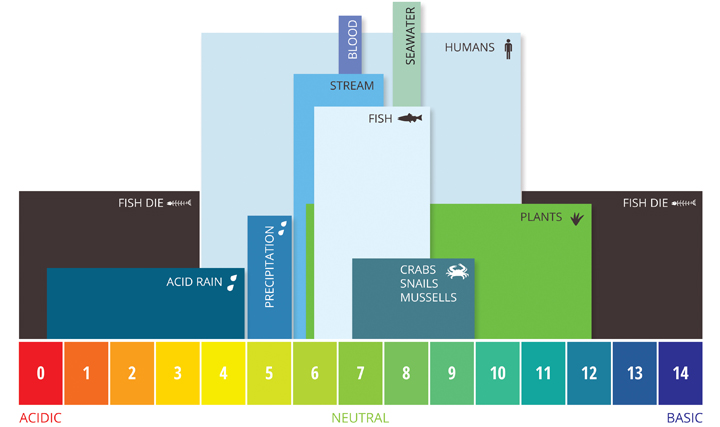
As pH levels move away from this range (upwards or down) it can stress brute systems and reduce hatching and survival rates. The further outside of the optimum pH range a value is, the higher the mortality rates. The more sensitive a species, the more than afflicted it is by changes in pH. In addition to biological furnishings, extreme pH levels usually increment the solubility of elements and compounds, making toxic chemicals more than "mobile" and increasing the risk of absorption by aquatic life ¹³.
Aquatic species are not the just ones affected by pH. While humans have a higher tolerance for pH levels (drinkable levels range from 4-eleven with minimal gastrointestinal irritation), there are notwithstanding concerns ¹⁴. pH values greater than eleven tin can cause skin and eye irritations, as does a pH below iv. A pH value below 2.5 volition cause irreversible harm to pare and organ linings ¹⁴. Lower pH levels increase the risk of mobilized toxic metals that can be absorbed, fifty-fifty past humans, and levels above viii.0 cannot be effectively disinfected with chlorine, causing other indirect risks ¹⁴. In addition, pH levels outside of 6.five-9.v can damage and corrode pipes and other systems, farther increasing heavy metal toxicity.
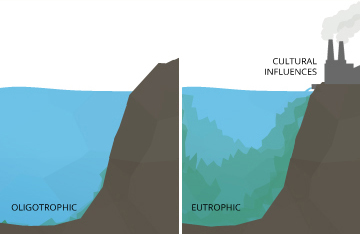
Even minor pH changes can have long-term effects. A slight modify in the pH of water can increase the solubility of phosphorus and other nutrients – making them more accessible for plant growth ¹⁰. In an oligotrophic lake, or a lake low in plant nutrients and high in dissolved oxygen levels, this can cause a concatenation reaction. With more accessible nutrients, aquatic plants and algae thrive, increasing the demand for dissolved oxygen. This creates a eutrophic lake, rich in nutrients and constitute life just low in dissolved oxygen concentrations. In a eutrophic lake, other organisms living in the water will become stressed, even if pH levels remained within the optimum range.
Factors that Influence the pH of Water
There are many factors that can affect pH in h2o, both natural and human-made. Most natural changes occur due to interactions with surrounding rock (peculiarly carbonate forms) and other materials. pH can also fluctuate with precipitation (especially acid pelting) and wastewater or mining discharges ¹³. In add-on, CO2 concentrations can influence pH levels.
Carbon Dioxide and pH
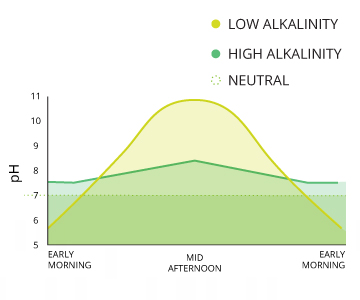
Carbon dioxide is the most mutual crusade of acidity in water ¹⁵. Photosynthesis, respiration and decomposition all contribute to pH fluctuations due to their influences on CO2 levels. The extremity of these changes depends on the alkalinity of the water, but there are often noticeable diurnal (daily) variations ¹⁶. This influence is more measurable in bodies of water with loftier rates of respiration and decomposition.
While carbon dioxide exists in water in a dissolved state (similar oxygen), it can besides react with water to grade carbonic acid:
CO2 + Water <=> H2CO3
H2CO3 tin can and then lose one or both of its hydrogen ions:
H2CO3 <=> HCO3– + H+ …. HCO3– <=> CO32- + H+
The released hydrogen ions subtract the pH of water¹⁵. Nonetheless, this equation can operate in both directions depending on the current pH level, working as its own buffering system. At a college pH, this bicarbonate arrangement volition shift to the left, and CO32- will choice up a free hydrogen ion.
This reaction is normally minimal as H2CO3 has a low solubility constant (Henry'south Law) ¹⁵. However, as CO2 levels increase around the world, the amount of dissolved CO2 as well increases, and the equation will be carried out from left to right. This increases H2CO3, which decreases pH. The effect is becoming more evident in oceanic pH studies over time.
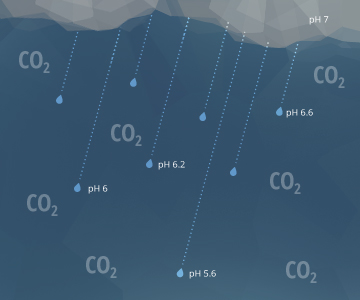
The to a higher place equations too explain why pelting has a pH of approximately 5.65 ¹⁵. As raindrops fall through the air, they interact with carbon dioxide molecules in the atmosphere. This creates H2CO3 in the raindrops, lowering the rain's pH value ¹⁷. A pH level of 5.65, though acidic, is non considered acid rain. Natural, unpolluted pelting or snow is expected to take pH levels near 5.6, assuming a standard atmospheric CO2 concentration of 0.0355% ¹⁵. Acid pelting requires a pH below 5.0 ²¹.
5.65 is also the pH of water that has equilibrated with the air and has non come in contact with carbonate materials or limestone.
Natural pH Influences
Carbonate materials and limestone are two elements that tin buffer pH changes in water. Calcium carbonate (CaCO3) and other bicarbonates can combine with both hydrogen or hydroxyl ions to neutralize pH¹⁸. When carbonate minerals are present in the soil, the buffering chapters (alkalinity) of h2o is increased, keeping the pH of water close to neutral even when acids or bases are added. Boosted carbonate materials beyond this tin can brand neutral water slightly basic.


As mentioned earlier, unpolluted pelting is slightly acidic (pH of 5.half-dozen). The pH of pelting can also be lowered due to volcanic ash, sulfate-reducing bacteria in wetlands, airborne particulates from wildfires and even lightning ¹⁹. If rain falls on a poorly buffered water source, it can decrease the pH of nearby water through runoff.

Pine or fir needles can also decrease the pH of soil, and any water that runs over it, equally they decompose ¹⁸. Intense photosynthesis increases the pH of water as it removes CO2, though this change is usually diurnal ²⁰.
Man-Made pH Influencers
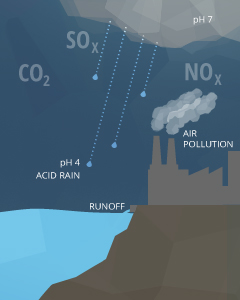
Anthropogenic causes of pH fluctuations are commonly related to pollution. Acid rain is one of the best known examples of human influence on the pH of water. Any form of atmospheric precipitation with a pH level less than 5.0 is known as acrid rain ²¹. This precipitation comes from the reaction of water with nitrogen oxides, sulfur oxides and other acidic compounds, lowering its already slightly acidic pH. These emissions unremarkably come from mining and smelting operations or fossil fuel combustion (coal burning and automobiles) ¹⁸. Extremely loftier levels of CO2 can as well farther decrease the pH of pelting ¹⁷.
Point source pollution is a common cause that can increase or decrease pH depending on the chemicals involved ¹⁸. These chemicals can come from agricultural runoff, wastewater discharge or industrial runoff. Mining operations (particularly coal) produce acid runoff and acidic groundwater seepage if the surrounding soil is poorly buffered ²². Wastewater belch that contains detergents and lather-based products can cause a water source to become also basic.
Typical pH Levels
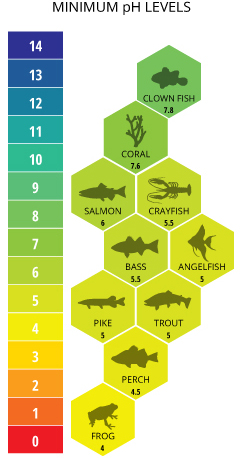
Typical pH levels vary due to ecology influences, particularly alkalinity. The alkalinity of water varies due to the presence of dissolved salts and carbonates, besides as the mineral composition of the surrounding soil. In general, the higher the alkalinity, the college the pH; the lower the alkalinity, the lower the pH ⁶. The recommended pH range for near fish is betwixt six.0 and ix.0 with a minimum alkalinity of twenty mg/L, with ideal CaCO3 levels between 75 and 200 mg/L ²⁰.
Oceanic organisms like clownfish and coral require higher pH levels. pH levels below 7.six volition crusade coral reefs to begin to collapse exercise to the lack of calcium carbonate ³⁹. Sensitive freshwater species such as salmon prefer pH levels between seven.0 and eight.0, becoming severely distressed and suffering physiological damage due to absorbed metals at levels beneath 6.0 ⁴⁰.
Environmental Considerations
Natural atmospheric precipitation, both rain and snow, has a pH near 5.6 due to contact with CO2 and other atmospheric influences. Most grasses and legumes adopt soils with a pH of 4.5-vii.0, and so the slight acidity of rain can do good carbonate soils ²³.
The acerbity of the surrounding environment tin can also bear on the pH of water. This is most obvious virtually mining areas, but the effect tin can besides occur naturally. Acrid runoff depletes the h2o's alkalinity and lowers pH beneath optimum levels. This may be tolerable for some aquatic species (such as frogs) but not for most fish. Some frogs and other amphibians can ofttimes tolerate pH levels as low as 4.0 ²⁴. Acidic soils in the Amazon cause many of the lakes and rivers to naturally have low pH values ³⁸. Due to the dissolved humic substances from runoff and uptake, "blackwater" sources tin can have a pH as low equally 4.43. "Clearwater" sources will take a slightly higher, but still acidic, pH value ³⁸. That is why angel fish and discus from the Amazon River Bowl can thrive quite happily in waters with a pH every bit depression as five.0 ²⁵.
Seawater has a pH around 8.2, though this can range between 7.5 to 8.v depending on its local salinity. pH levels will increase with salinity until the water reaches calcium carbonate (CaCO3) saturation ¹⁶. The oceans by and large have a higher alkalinity due to carbonate content and thus have a greater ability to buffer free hydrogen ions ²⁷.
Freshwater lakes, ponds and streams usually have a pH of vi-8 depending on the surrounding soil and boulder ²¹. In deeper lakes where stratification (layering) occurs, the pH of water is by and large higher (7.v-8.5) near the surface and lower (half dozen.5-seven.5) at greater depths ¹⁰. Some states, such every bit Alaska, are attempting to maintain a pH standard for h2o quality. The Alaska Water Quality Standard requires pH levels betwixt 6.5 and viii.5 to protect the many salmon populations in the land ⁴⁰.
Stratification Considerations
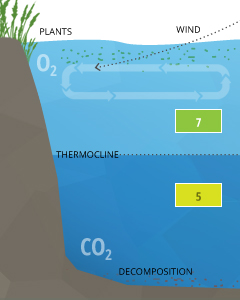
Stratification is usually caused by temperature differences within a body of water, where each layer of water does not mix with the layers higher up or below ³⁷. These layers are separated by clines, known as thermoclines (temperature divides) or chemoclines (chemistry gradients). Chemoclines tin exist based on oxygen, salinity, or other chemical factors that practise non cross the cline, such every bit carbon dioxide. Due to CO2'south influence on the pH of h2o, stratification tin cause pH levels to differ beyond a cline.
Differences in pH levels betwixt water strata are due to increased CO2 from respiration and decomposition below the thermocline. In crater lakes such as Lake Nyos or Lake Monoun, the pH rapidly drops from a surface level around vii to v.five below 60 m (at the thermocline and chemocline) ²⁶. This significant driblet comes from the saturated CO2 that is stored up in the lower strata of the lake.
Adaptability
While platonic pH levels for fish are 7-8 (fish blood has a pH of 7.4) ²⁰, nigh fish tin can adjust to the pH level of their environment (half dozen.0-9.0) as long as in that location are no dramatic fluctuations. A dramatic fluctuation is considered a shift in pH of 1.iv (up or down) ²². For saltwater fish, the pH of water should remain between vii.5 and viii.5 ⁹.
Unusual pH Levels and Consequences

Harmful furnishings get noticeable when the pH of water falls below five.0 or rising above nine.6. Sick effects due to acidification are more pronounced in saltwater fish due to their accommodation to a higher pH. When pH is below optimal levels, fish become susceptible to fungal infections and other concrete impairment ¹⁶. As the pH of water falls, the solubility of calcium carbonate is reduced, inhibiting shell growth in aquatic organisms ¹⁶. In general, fish reproduction is affected at pH levels beneath 5.0 and many species (such as saltwater fish or sensitive freshwater fish similar smallmouth bass) will exit the surface area ²¹. Fish begin to dice when pH falls below 4.0 ¹².
Low pH levels tin encourage the solubility of heavy metals ¹². As the level of hydrogen ions increases, metal cations such as aluminum, lead, copper and cadmium are released into the water instead of being absorbed into the sediment. Every bit the concentrations of heavy metals increment, their toxicity as well increases. Aluminum can limit growth and reproduction while increasing mortality rates at concentrations as depression equally 0.1-0.3 mg/L ²². In improver, mobilized metals tin exist taken in by organisms during respiration, causing physiological damage ²² . This is particularly detrimental to species such equally rainbow trout ¹³.
On the other side of the spectrum, high pH levels can damage gills and peel of aquatic organisms and cause decease at levels over 10.0. While some african cichlids thrive at high pH levels (upwardly to nine.5), most fish cannot tolerate them. Death can occur even at typical levels (9.0) if ammonia is nowadays in the h2o ²¹. At depression and neutral pH levels, ammonia combines with h2o to produce an ammonium ion:
NH3 + H2O <=> NH4+ + OH–

Ammonium, NH4⁺, is non-toxic and will not affect aquatic life. Yet, at pH levels over 9, the equation reverses and ammonia is released into the water ²². Ammonia, NH3, is extremely toxic to aquatic organisms, and every bit pH increases, the mortality rates ascent with the NH3 concentration.
On the ecosystem side, mosses tin brainstorm invading a body of water as the pH of water falls below five. In eutrophic lakes, pH-tolerant algae can dominate, driving the pH levels to diurnal high and low extremes, forming algae blooms that can kill the lake ¹⁶.
Alkali metal and Acid Lakes
Spread across the world are a number of lakes with unusual pH levels. Alkaline lakes, as well known equally soda lakes, generally accept a pH level between ix and 12. This is often due to a high salt content (though not every common salt lake has a high pH). These lakes have loftier concentrations of minerals, particularly dissolved salts: sodium, calcium, magnesium carbonates and bicarbonates ²⁸. Depending on the lake, borates, sulfates and other elements (usually strong base ions) can also be present ²⁹. Alkali metal lakes are formed when the only outlet for water is evaporation, leaving the minerals behind to accrue ³⁰. These minerals often form columns of mineral deposits, known every bit tufa columns. Many alkaline lakes are a commercial resource for soda ash and potash, while others are popular tourist destinations for their "magical" healing properties (due to the mineral content).
A notorious case of an alkaline metal lake is Lake Natron in Tanzania. Lake Natron has a pH up to 10.5 due to high concentrations of sodium carbonate decahydrate (soda ash) and sodium bicarbonate (baking soda) that enters the water from the surrounding soil ³¹. While the lake supports a thriving ecosystem, including flamingos, alkaline tilapia and pH-resistant algae, Nick Brant, a lensman, has created many haunting images of animals that died in this lake ³¹. The bodies of these animals are preserved by the sodium carbonate, much similar the ancient Egyptian mummification process.
Acid lakes usually develop near volcanoes, where sulfuric acid, hydrogen sulfide, hydrofluoric acid, hydrochloric acid and carbon dioxide can leach into the h2o ³². In non-volcanic areas, acid lakes tin can also develop after acidic deposition from events such as acid rain, pollution or acid runoff from mining operations ³³. Much like their alkaline counterparts, acid lakes have no outlet except evaporation, concentrating the sulfates and acids. The acids can enter the water through atmospheric diffusion from coal burning, acid pelting or after an eruption. In volcanic lakes, acids can enter the h2o through an active fumarole, or volcanic vent.
The acid lakes at Dallol in Federal democratic republic of ethiopia are the result of acid leaching from nearby volcanoes. The sulfur and iron in the h2o leave yellow and rust-colored deposits around the water's edge.
With a pH level below v.0, few organisms can live in acid lakes. However, there is one notable exception: the Osorezan dace, or Japanese dace. This fish thrives in the acidic waters of Lake Osorezan, resting comfortably at a pH of 3.5, and swims into neutral pH waters merely to spawn ³⁴.
Bounding main Acidification
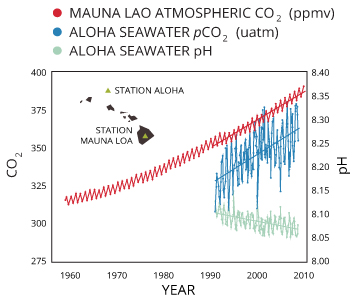
Sea acidification is caused by an influx of dissolved carbon dioxide. Equally atmospheric CO2 levels increase due to anthropogenic causes, dissolved CO2 also increases, which in turn decreases the pH of water.
When water becomes saturated with CO2, it not simply reduces the ocean'due south pH, but depletes the calcium carbonate sources also ³⁵. Calcium carbonate, CaCO3, is a necessary ingredient in building corals, shells and exoskeletons for many aquatic creatures. As CO3²⁻ levels decrease, information technology becomes more hard for marine creatures to build their shells.
As mentioned in the section "Carbon Dioxide and pH", boosted CO2 increases the number of hydrogen ions in the water, reducing pH:
CO2 + H2O <=> H2CO3 … H2CO3 <=> (H+) + HCO3⁻
At pH levels between six.4 and x.33, some of those hydrogen ions attach to carbonate ions ²²:
(H+) + CO3two- <=> HCO3–
Thus as CO2 levels increase, the availability of carbonate, CO32- decreases, reducing the amount bachelor for shell and coral building ³⁶.
CO2 + H2O + CO3²⁻ <=> 2HCO3⁻
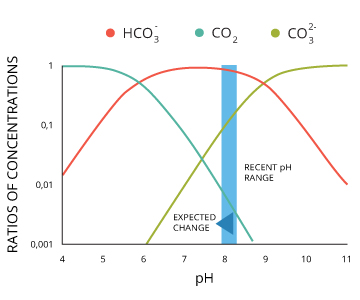
High CO2 levels also brand it more difficult to maintain electric current shells due to lower pH levels and competition for carbonate ³⁵.
Furthermore, the air saturation of water is based on fractional pressures from Henry's law. Every bit CO2 levels in the air increase, so too does their partial pressure. This reduces the partial force per unit area of oxygen, reducing its saturation levels and contributing to hypoxic (depression O2) weather ³⁵.
While the oceans will never become "acidic" (with a pH of less than seven), even decreasing pH a slight amount stresses saltwater organisms and increases mortality rates. pH is logarithmic, meaning that a decrease past 0.one is equivalent to virtually a 30% increase in acidity ³⁵.
Cite This Work
Fondriest Ecology, Inc. "pH of Water." Fundamentals of Environmental Measurements. 19 Nov. 2013. Web. < https://www.fondriest.com/ecology-measurements/parameters/water-quality/ph/ >.
More than Information
- pH Measurement Methods
- pH Meters
- pH Sensors
- Applications
- References
santanayoughlythers.blogspot.com
Source: https://www.fondriest.com/environmental-measurements/parameters/water-quality/pH/
0 Response to "what is a kill in reference to water"
Post a Comment